Class 11 Chemistry Structure of Atom MCQs with Answers
Class 11 Chemistry Structure of Atom MCQs with Answers offer a targeted and comprehensive tool for Sainik School aspirants preparing for their chemistry exams. These multiple-choice questions are specifically tailored to the class 11 chemistry syllabus, focusing on the fundamental topic of the structure of the atom. With clear and concise answers provided, students can efficiently self-assess, identifying strengths and areas for improvement. A solid understanding of atomic structure is crucial, and these MCQs ensure thorough comprehension of essential concepts.
As students gear up for Sainik School entrance exams, practicing these MCQs not only aids in exam readiness but also enhances critical thinking and problem-solving skills. The content aligns with the broader goals of Sainik School education, emphasizing a holistic understanding of atomic structure principles. This resource serves as a strategic tool, helping students build a strong foundation for future academic endeavors and meet the specific requirements of Sainik School examinations
Q1. Number of angular nodes for 4d orbital is _.
- 4
- 3
- 2
- 1
Click To View The Answer
Ans: 3. 2
Q2. Total number of orbitals associated with third shell will be __.
- 2.
- 4.
- 9.
- 3.
Click To View The Answer
Ans: 3. 9.
Explanation:
No of orbitals in 3 shell (n = 3) = n = 3 = 9.
Q3. The radius of second Bohr’s orbits for hydrogen atoms is:
h = 6.6262 × 10−34Js, me = 9.109 × 10−31kg, e change = 1.6021 × 1019C
- 1.65A˚
- 4.76A˚
- 0.529A˚
- 2.12A˚
Click To View The Answer
Ans:4. 2.12A˚
Q4. The number of radial nodes for 3p orbital is __.Q60. The number of radial nodes for 3p orbital is __.
- 3
- 4
- 2
- 1
Click To View The Answer
Ans: 4. 1
Explanation:
For a hydrogen atom wave function, there are n – l – 1 radial nodes and (n – 1) total nodes. Number of radial nodes for 3p orbital = n – l
- 1
= 3 – 1 – 1 = 1
Q5. The radius of which of the following orbit is same as that of first orbit of hydrogen atom?
- He+(n = 2)
- L2+(n = 2)
- Li2+(n = 3)
- Be3+(n = 2)
Click To View The Answer
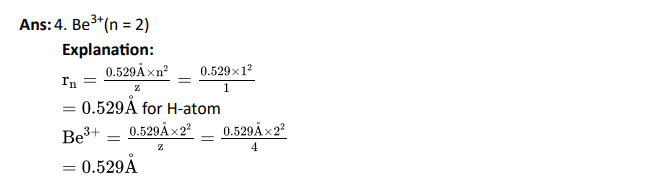
Q6. On bombarding a beam of a-particles on the atom of the gold sheet, a few particles get deflected whereas most
of them go straight and remains undeflected. This is due to:
- The nucleus occupy much smaller volume as compared to the volume of atom.
- The force of repulsion on fast moving a-particles is very small.
- The neutrons in the nucleus do not have any effect on a-particles.
- The force of attraction on a-particles by the oppositely charged electron is not sufficient.
Click To View The Answer
Ans: 1. The nucleus occupy much smaller volume as compared to the volume of atom.
Explanation:
On bombarding a beam of a-particles on the atom of the gold sheet, a few particles get deflected whereas most of them go straight
and remains undeflected because the nucleus occupy much smaller volume as compared to the volume of atom.
Q7. Electronic configuration of five elements I, II, III, IV, V is mentioned below.
In the above configuration element I, II, III, IV and V represent as:
- C, N, O, F, Ne
- Ne, F, O, N, C
- C, O, N, Ne, F
- O, C, F, Ne, V
Click To View The Answer
Ans: 1. C, N, O, F, Ne
Q8. The probability density plots of 1s and 2s orbitals are given in figure.
The density of dots in a region represents the probability density of finding electrons in the region.
On the basis of above diagram which of the following statements is incorrect?
- 1s and 2s orbitals are spherical in shape.
- The probability of finding the electron is maximum near the nucleus.
- The probability of finding the electron at a given distance is equal in all directions.
- The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus
increases.
Click To View The Answer
Ans: 4. The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases.
Explanation:
The probability density of electrons in 2s orbital first increases then decreases and after that it begins to increases again as distance
increases from nucleus.
Q9. The correct set of four quantum numbers for the valence electrons of rubidium atom (Z = 37) is:

Click To View The Answer

Q10. Which of the following is responsible to rule out the existence of definite paths or trajectories of electrons?
- Pauli’s exclusion principle.
- Heisenberg’s uncertainty principle.
- Hund’s rule of maximum multiplicity.
- Aufbau principle.
Click To View The Answer
Ans: 2. Heisenberg’s uncertainty principle.
Q11. In atom, an electron is moving with a speed of 600m/ s with an accuracy of 0.005%. Certainty with which the
position of the electron can be located is:
(h = 6.6 × 10-34kg/ m2s-1, mass of electron, em = 6.6 × 10-31kg)
1. 1.52 × 10-4m
2. 5.10 × 10-3m
3. 1.92 × 10-3m
4. 3.84 × 10-3m
Click To View The Answer
Ans:3. 1.92 × 10-3m
Q12. A ray of white light is spread out into a series of coloured bands of visible light are called:
- Visible band.
- Spectrum.
- Electronic spectrum.
- None of these.
Click To View The Answer
Ans: 2. Spectrum.
Q13. Identify the pairs which are not of isotopes?

Click To View The Answer

Q14. If EA, EB and EC represent kine c energies of an electron, alpha par cle and proton respec vely and each moving with same de-Broglie wavelength, then choose the correct increasing representa on,
1. EA = EB = EC
2. EA > EB > EC
3. EB > EC > EA
4. EA < EC < EB
Click To View The Answer
Ans:4. EA < EC < EB
Q15. Which of the following is the energy of a possible excited state of hydrogen?
- +13.6eV
- -6.8eV
- -3.4eV
- +6.8eV
Click To View The Answer
Ans: 3. -3.4eV
FAQs
Q: How can Class 11 Chemistry Structure of Atom MCQs with Answers assist Sainik School aspirants in their exam preparation?
A: Engaging with Structure of Atom MCQs designed for class 11 ensures that Sainik School candidates have a focused study resource. These questions cover key concepts related to the atomic structure and provide clear answers, facilitating efficient self-assessment. By practicing these MCQs, students not only reinforce their understanding but also develop strong problem-solving skills, crucial for success in the competitive Sainik School entrance exams. The focus on the structure of the atom aligns with the curriculum, ensuring that aspirants are well-prepared for the specific content they will encounter in the examinations.
Q: How does the inclusion of Class 11 Chemistry Structure of Atom MCQs contribute to a comprehensive education approach at Sainik School?
A: Integrating Structure of Atom MCQs into the study material reflects Sainik School’s commitment to holistic education. Beyond exam preparation, these questions foster a deeper understanding of atomic structure principles, contributing to a well-rounded education. The clear answers provided not only help in grasping theoretical concepts but also promote effective problem-solving skills. This aligns with Sainik School’s broader goal of nurturing individuals with a comprehensive academic foundation, ensuring that students are not only successful in the entrance exams but also well-equipped for future academic pursuits.
