Class 11 Chemistry States of Matter MCQs with Answers
Class 11 Chemistry States of Matter MCQs with Answers serve as a targeted and invaluable resource for Sainik School aspirants preparing for their chemistry exams. These multiple-choice questions are meticulously tailored to the class 11 chemistry syllabus, focusing on the fundamental topic of states of matter. With clear and concise answers provided, students benefit from efficient self-assessment, identifying strengths and areas for improvement. Mastering states of matter is pivotal in understanding various chemical processes, and these MCQs ensure a thorough comprehension of essential concepts.
As students gear up for Sainik School entrance exams, practicing these MCQs not only aids in exam readiness but also enhances critical thinking and problem-solving skills. The content aligns with the broader goals of Sainik School education, emphasizing a holistic understanding of states of matter principles. This resource serves as a strategic tool, helping students build a strong foundation for future academic endeavors and meeting the specific requirements of Sainik School examinations.
Q1. The pressure exerted by the gaseous molecules is due to:
- The repulsion between gaseous molecules.
- The attraction between gaseous molecules.
- Collision of the particles with the walls of the container.
- Both (a) and (b).
Click To View The Answer
Ans: 3. Collision of the particles with the walls of the container.
Q2. The gases are easily liquefied when:
- They are compressed at higher temperature.
- They are compressed at lower temperature.
- They are expanded at higher temperature.
- They are expanded at lower temperature.
Q3. With regard to the gaseous state of matter which of the following statements are correct?
- Complete order of molecules.
- Complete disorder of molecules.
- Random motion of molecules.
- Fixed position of molecules.
Click To View The Answer
Ans: 2. Complete disorder of molecules.
- Random motion of molecules.
Q4. Critical temperature is the how so ever:
- Highest temperature above which gas cannot be liquefied.
- Highest temperature above which gas liquefies.
- Lowest temperature at which gas liquefies.
- All of the above.
Click To View The Answer
Ans: 1. Highest temperature above which gas cannot be liquefied.
Explanation:
Critical temperature is the highest temperature above which gas cannot be liquefied how so ever pressure is applied, i.e. above this
temperature it exists as a gas.
Q5. By what factor does the average velocity of a gaseous molecules increase when the temperature is doubled?
- 2
- 2.8
- 4.0
- 1.4
Click To View The Answer
Ans: 4. 1.4
Q6. If Z is a compressibility factor, van der Waals’ equation at low pressure can be written as:

Click To View The Answer
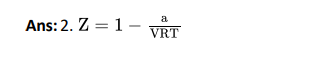
Q7. If a gas expands at constant temperature, it indicates that:
- Kinetic energy of molecules decreases.
- Pressure of the gas increases.
- Kinetic energy of molecules remain the same.
- Number of molecules of gas increase.
Click To View The Answer
Ans: 3. Kinetic energy of molecules remain the same.
Q8. Critical temperature of H2O, NH3 , CO2 and O2 are 647K, 405.6K, 304.10K, and 154.2K respectively. If the cooling
starts from 500K to their critical temperature, the gas that liquefies first is:
- H2O
- NH3
- CO2
- O2
Click To View The Answer
Ans: 1. H2O
Explanation:
It will liquefies first due to maximum inter molecule forces.
Q9. The liquefaction behaviour of the temporary gases like CO2 approaches that of permanent gases like N2 , O2 etc.,
as we go:
- Below critical temperature.
- Above critical temperature.
- Above absolute zero.
- Below absolute zero.
Click To View The Answer
Ans: 2. Above critical temperature.
Explanation:
Critical temperature of a gas is highest temperature at which liquefaction of gas first occur. Liquefaction of permanent gases requires
cooling as well as considerable compression.
Q10. All gases obey Charle’s law at:
- Low temperature and high pressure.
- Low temperature and low pressure.
- High temperature and high pressure.
- High temperature and low pressure.
Click To View The Answer
Ans: 4. High temperature and low pressure.
Explanation:
All gases obey Charle’s law at very low pressures and high temperatures.
Q11. The density of gas is 1.964g cm-3 at 273K and 76cm Hg. The gas is:
- Xe
- CO2
- C2 H6
- CH4
Click To View The Answer
Ans: 2. CO2
Q12. Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of dipoles possess ‘partial
charges’. The partial charge is:
- More than unit electronic charge.
- Equal to unit electronic charge.
- Less than unit electronic charge.
- Double the unit electronic charge.
Click To View The Answer
Ans: 3. Less than unit electronic charge.
Q13. The average kinetic energy per molecule of a gas at a given temperature, T is given by:

Click To View The Answer

Q14. The pressure in well inflated tyres of automobiles is almost constant, but on a hot summer day this increases
considerably and tyre may burst. This phenomena is explained by:
- Boyle’s law.
- Charle’s law.
- Gay Lussac’s law.
- Avogadro’s law.
Click To View The Answer
Ans: 3. Gay Lussac’s law.
Q15. The surface tension of which of the following liquid is maximum?
- C2 H5 OH
- CH3 OH
- H2 O
- C6 H6
Click To View The Answer
Ans.3.H2 O
FAQS
Q: How do Class 11 Chemistry States of Matter MCQs with Answers contribute to effective preparation for Sainik School entrance exams?
A: Engaging with States of Matter MCQs designed for class 11 ensures Sainik School aspirants have a focused study approach. These questions cover essential concepts related to states of matter and provide clear answers, facilitating efficient self-assessment. By practicing these MCQs, students not only reinforce their understanding but also develop strong problem-solving skills, essential for success in the competitive Sainik School entrance exams. The emphasis on states of matter aligns with the curriculum, ensuring that aspirants are well-prepared for the specific content they will encounter in the examinations.
Q: How does the inclusion of Class 11 Chemistry States of Matter MCQs support a comprehensive education approach at Sainik School?
A: Incorporating States of Matter MCQs into the study material reflects Sainik School’s commitment to holistic education. Beyond exam preparation, these questions foster a deeper understanding of states of matter principles, contributing to a well-rounded education. The clear answers provided not only help in grasping theoretical concepts but also promote effective problem-solving skills. This aligns with Sainik School’s broader goal of nurturing individuals with a comprehensive academic foundation, ensuring that students are not only successful in the entrance exams but also well-equipped for future academic pursuits.
