Class 12 Chemistry Polymers MCQs with Answer
At Sainik School, our Class 12 Chemistry MCQs on “Polymers” offer students a comprehensive platform to master this essential topic. Covering various aspects of polymers, including types of polymers, polymerization methods, properties, and applications, these multiple-choice questions provide thorough preparation for Class 12 Chemistry exams.
Our MCQs challenge students to apply their knowledge of polymer chemistry to solve problems and analyze scenarios, fostering critical thinking and analytical skills essential for success in exams. Each MCQ is accompanied by a detailed answer explanation, enabling students to grasp the underlying concepts thoroughly.
By practicing Class 12 Chemistry MCQs on “Polymers” at Sainik School, students not only prepare effectively for their exams but also gain a deeper understanding of the principles governing polymerization, structure-property relationships, and the diverse applications of polymers in various industries, laying a strong foundation for their future studies and careers in chemistry and related fields.
Q1. Which of the following statements is not true about low density polythene?
- Tough.
- Hard.
- Poor conductor of electricity.
- Highly branched structure.
Click To View The Answers
Ans: 2. Hard.
Explanation:
Low density polythene: It is obtained by the polymerisation of ethane under high pressure of 1000 to 2000 atmosphere at a
temperature of 350K to 570K in the presence of traces of dioxygen or a peroxide initiator (catalyst). They are flexible in nature
Q2. Which of the following polymers are thermoplastic?
- Teflon.
- Natural rubber.
- Neoprene.
- Polystyrene.
Click To View The Answers
Ans: 1. Teflon.
- Polystyrene.
Explanation:
Teflon and polystyrene are thermoplastics since they can be moulded again by heating and melting.
Q3. Which of the following are addition polymers?
- Nylon.
- Melamine formaldehyde resin.
- Orlon.
- Polystyrene.
Click To View The Answers
Ans: 1. Nylon.
- Polystyrene.
Q4. Which of the following polymers are used as fibre?
- Polytetrafluoroethane.
- Polychloroprene.
- Nylon.
- Terylene.
Click To View The Answers
Ans: 3. Nylon.
- Terylene.
Q5. Vulcanisation makes rubber __.
- More elastic.
- Soluble in inorganic solvent.
- Crystalline.
- More stiff.
Click To View The Answers
Ans: 1. More elastic.
- More stiff.
Q6. The commercial name of polyacrylonitrile is _.
- Dacron.
- Orlon (acrilan).
- PVC.
- Bakelite.
Click To View The Answers
Ans: 2. Orlon (acrilan)
Q7. Which of the following polymers, need atleast one diene monomer for their preparation?
- Dacron.
- Buna-S.
- Neoprene.
- Novolac.
Click To View The Answers
Ans: 2. Buna-S.
- Neoprene.
Q8. Which of the following polymers of glucose is stored by animals?
- Cellulose.
- Amylose.
- Amylopectin.
- Glycogen.
Click To View The Answers
Ans: 4. Glycogen.
Explanation:
Glycogen is a polymer of glucose found in lever, brain and muscles of animals.
Q9. Which of the following polymers are condensation polymers?
- Bakelite.
- Teflon.
- Butyl rubber.
- Melamine formaldehyde resin.
Click To View The Answers
Ans: 1. Bakelite.
Q10. Which of the following polymer can be formed by using the following monomer unit?
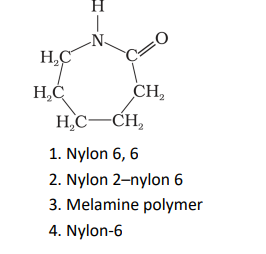
Click To View The Answers
Ans: 4. Nylon-6
Explanation:
It is obtained by heating caprolactum with water at a high temperature.
Q11. Which of the following polymers have vinylic monomer units?
- Acrilan.
- Polystyrene.
- Nylon.
- Teflon.
Click To View The Answers
Ans: 1. Acrilan.
- Polystyrene.
- Teflon.
Q12. Which of the folloiwng are characteristics of thermosetting polymers?
- Heavily branched cross linked polymers.
- Linear slightly branched long chain molecules.
- Become infusible on moulding so cannot be reused.
- Soften on heating and harden on cooling, can be reused.
Click To View The Answers
Ans: 1. Heavily branched cross linked polymers.
- Become infusible on moulding so cannot be reused.
Q13. Which of the following are example of synthetic rubber?
- Polychloroprene.
- Polyacrylonitrile.
- Buna-N.
- cis-polyisoprene.
Click To View The Answers
Ans: 1. Polychloroprene.
- Buna-N.
Q14. Which of the following monomers form biodegradable polymers?
- 3-hydroxybutanoic acid + 3-hydroxypentanoic acid.
- Glycine + amino caproic acid.
- Ethylene glycol + phthalic acid.
- Caprolactum.
Click To View The Answers
Ans: 1. 3-hydroxybutanoic acid + 3-hydroxypentanoic acid.
- Glycine + amino caproic acid.
Q15. Which of the following is not a semisynthetic polymer?
- Cis-polyisoprene.
- Cellulose nitrate.
- Cellulose acetate.
- Vulcanised rubber.
Click To View The Answers
Ans: 1. Cis-polyisoprene.
Explanation:
Cis-polyisoprene is not a semisynthetic polymer whereas other is semisynthetic polymers.
FAQs
How do MCQs on “Polymers” in Class 12 Chemistry help students understand the classification and properties of different types of polymers?
MCQs on “Polymers” play a crucial role in aiding Class 12 Chemistry students to comprehend the classification, properties, and characteristics of various types of polymers. These MCQs cover topics such as addition polymerization, condensation polymerization, polymer properties, polymerization techniques, and polymer applications. By engaging with these MCQs, students can deepen their understanding of the structure-property relationships specific to different types of polymers, enabling them to analyze and solve problems related to polymer chemistry effectively.
What strategies can students employ to effectively prepare for Class 12 Chemistry exams using MCQs on “Polymers”?
To effectively prepare for exams using MCQs on “Polymers,” students should first ensure a solid understanding of the fundamental concepts and classification schemes within polymer chemistry. They can then practice solving a variety of MCQs from reputable sources, focusing on different aspects such as polymerization methods, polymer properties, and polymer applications. It’s essential to analyze both correct and incorrect answers to deepen understanding and identify areas for improvement. Additionally, students can use MCQs to simulate exam conditions, helping them become familiar with the format and timing of the actual examination. Regular practice with MCQs, coupled with comprehensive revision of concepts, can significantly enhance students’ performance in Class 12 Chemistry exams.
