Class 12 The P-block Elements MCQs with Answers
Sainik School offers Class 12 Chemistry MCQs focusing on “P-block Elements” to aid students in mastering this essential topic. These multiple-choice questions cover a comprehensive range of aspects related to p-block elements, encompassing Group 13 to Group 18 elements, their properties, compounds, and industrial applications.
By engaging with these MCQs, students can deepen their understanding of p-block elements’ characteristics and their significance in various fields of chemistry and industry. Each MCQ is accompanied by accurate answer explanations, facilitating thorough comprehension and self-assessment.
Practicing Class 12 Chemistry MCQs on “P-block Elements” at Sainik School not only prepares students effectively for their exams but also equips them with essential analytical and problem-solving skills. Mastery of p-block elements is crucial for a strong foundation in chemistry, benefiting students in their academic pursuits and future careers in science and technology.
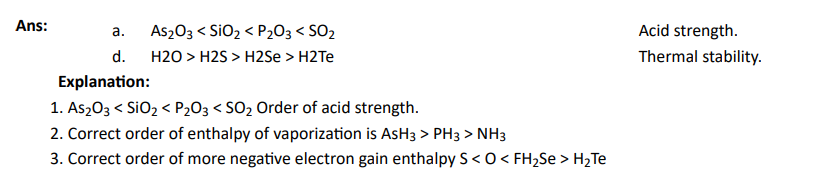
Q1. Which of the following options are not in accordance with the property mentioned against them?

Click To View The Answers

Q2. Which of the following orders are correct as per the properties mentioned against each?

Click To View The Answers
Q3. Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.
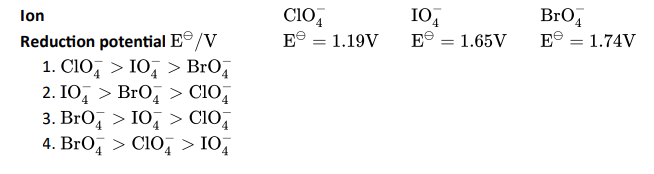
Click To View The Answers


Click To View The Answers

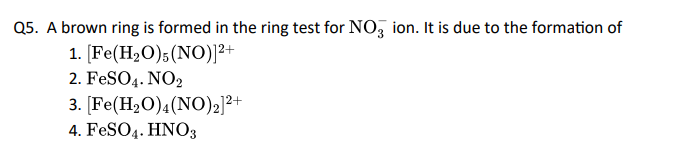
Click To View The Answers

Q6. Which of the following statements are correct?

Click To View The Answers
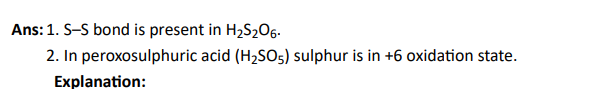
Q7. In the preparation of HNO3 , we get NO gas by catalytic oxidation of ammonia. The moles of NO produced by the
oxidation of two moles of NH3 will be __.
- 2
- 3
- 4
- 6
Click To View The Answers
Ans: 1. 2

Q8.
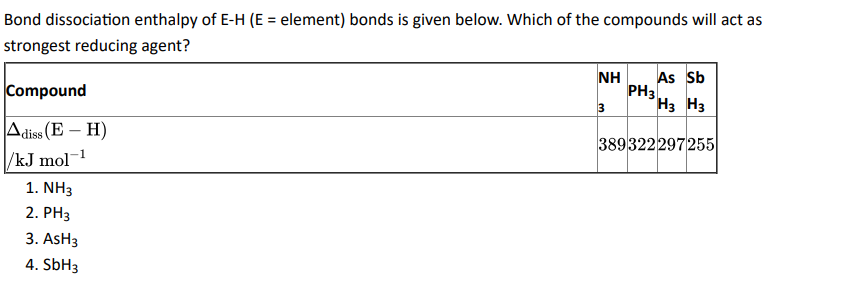
Click To View The Answers
Ans: 4. SbH3
Q9. Which of the following statements are true?
- Only type of interactions between particles of noble gases are due to weak dispersion forces.
- Ionisation enthalpy of molecular oxygen is very close to that of xenon.
- Hydrolysis of XeF6 is a redox reaction.
- Xenon fluorides are not reactive.
Click To View The Answers
Ans: 1. Only type of interactions between particles of noble gases are due to weak dispersion forces.
- Ionisation enthalpy of molecular oxygen is very close to that of xenon.
Q10. On heating with concentrated NaOH solution in an inert atmosphere of CO2 , white phosphorus gives a gas. Which
of the following statement is incorrect about the gas?
- It is highly poisonous and has smell like rotten fish.
- It’s solution in water decomposes in the presence of light.
- It is more basic than NH3 .
- It is less basic than NH3 .
Click To View The Answers
Ans: 3. It is more basic than NH3 .
Q11. Which of the following elements does not show allotropy?
- Nitrogen.
- Bismuth.
- Antimony.
- Arsenic.
Click To View The Answers
Ans: 1. Nitrogen.
Q12. The oxidation state of central atom in the anion of compound NaH2PO2 will be __
- +3
- +5
- +1
- -3
Click To View The Answers
Ans: 3. +1
Q13. In which of the following reactions conc. H2 SO4 is used as an oxidising reagent?
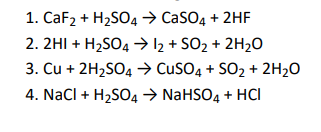
Click To View The Answers
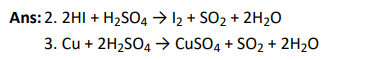
Q14. Which of the following statements are correct?
- All the three N—O bond lengths in HNO3 are equal.
- All P—Cl bond lengths in PCl5 molecule in gaseous state are equal.
- P4 molecule in white phohsphorus have angular strain therefore white phosphorus is very reactive.
- PCl is ionic in solid state in which cation is tetrahedral and anion is octahedral.
Click To View The Answers
Ans: 3. P4 molecule in white phohsphorus have angular strain therefore white phosphorus is very reactive.
- PCl is ionic in solid state in which cation is tetrahedral and anion is octahedral.
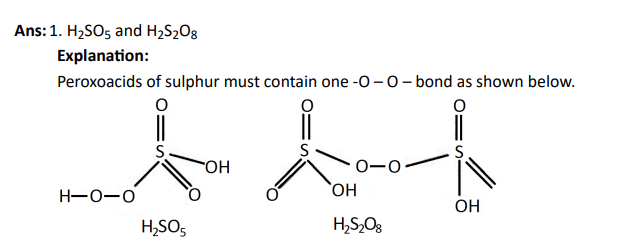
Q30. Which of the following are peroxoacids of sulphur?
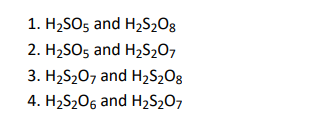
Click To View The Answers
FAQs
How do MCQs on “P-block Elements” in Class 12 Chemistry help students understand the unique properties and trends within the periodic table?
MCQs on “P-block Elements” play a vital role in aiding Class 12 Chemistry students to comprehend the diverse properties and trends exhibited by elements in the p-block of the periodic table. These MCQs cover topics such as atomic and molecular properties, chemical reactivity, and industrial applications of p-block elements from Group 13 to Group 18. By engaging with these MCQs, students can deepen their understanding of the periodic trends, electron configurations, and bonding characteristics unique to p-block elements, enabling them to analyze and solve problems related to these elements effectively.
How can students effectively utilize MCQs on “P-block Elements” for exam preparation?
To effectively prepare for exams using MCQs on “P-block Elements,” students should first ensure a solid understanding of the fundamental concepts and trends within the p-block of the periodic table. They can then practice solving a variety of MCQs from reputable sources, focusing on different aspects such as chemical properties, industrial applications, and comparative reactivity of p-block elements. It’s essential to analyze both correct and incorrect answers to deepen understanding and identify areas for improvement. Additionally, students can use MCQs to simulate exam conditions, helping them become familiar with the format and timing of the actual examination. Regular practice with MCQs, coupled with comprehensive revision of concepts, can significantly enhance students’ performance in Class 12 Chemistry exams.
